

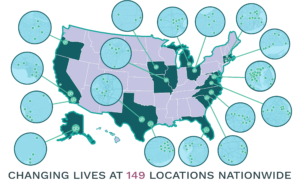
Greenbrook TMS (NASDAQ: GBNH, TSX: GTMS) is a leading provider of TMS therapy, an FDA-cleared, non-invasive therapy for the treatment of Major Depressive Disorder and other mental health disorders founded by Greybrook and firm’s CEO, Bill Leonard, with 149 centres across the U.S. The company has provided nearly 1,000,000 treatments changing the lives of more than 20,000 patients and has achieved phenomenal growth over the past year through clinic acquisitions, and expansions into new therapies and treatment methods.
Greenbrook TMS was founded with the belief that mental health care as it then existed was failing too many people. Only 49% of patients respond to their first-round antidepressant medication. The other 51% endured lengthy cycles of medication trial-and-error with little improvement, often experiencing side effects like weight gain and sexual dysfunction which worsened their depression symptoms. We knew there had to be a better way.
In 2011, Greenbrook TMS opened its first location in Tysons Corner, Virginia, with a vision to bring together the best care teams and technology into a more personalized, more accessible, and most importantly more effective treatment experience.
Today, more than 10 years after launching that first centre, Greenbrook has provided nearly 1,000,000 treatments and changed the lives of more than 20,000 patients. “We have the largest clinical data set in the U.S., and our TMS patients experience a 62% response rate which is well above the current antidepressant medication therapy rates and that is for patients who have already failed other treatments”, explained Bill Leonard, CEO Greenbrook TMS. “Many of our patients achieve full remission from their depression symptoms—all with little to no side effects”. With more than 40% growth since 2015, Greenbrook’s 149 centers span 17 states; each location offers state-of-the-art treatment technology in a soothing, spa-like environment, bringing the Greenbrook care experience to more communities. In early 2021, to complement Greenbrook’s deep expertise in depression treatment, the company piloted offering Spravato, a nasal esketamine spray that is FDA-approved for treatment resistant major depressive disorder.
“Our passion has always been fueled by the goal of changing patient’s lives for the better. Looking ahead, as the pandemic continues to disrupt all aspects of daily life, rates of depression continue to soar and the importance of and need for our work is greater than ever. To help meet treatment needs in the community, and building on our successes in 2021, we will expand our Spravato offering to 23 locations by the end of Q1 2022”.
“We are very proud of what the Greenbrook team has accomplished and their commitment to helping so many people. As we expand Greenbrook’s clinical footprint, we are excited to see the impact that TMS and other innovative therapies can have on the lives of patients struggling with mental health”, Elias Vamvakas, Chairman Greenbrook TMS and Chairman Greybrook Capital.
To learn more about Greenbrook TMS, visit www.greenbrooktms.com